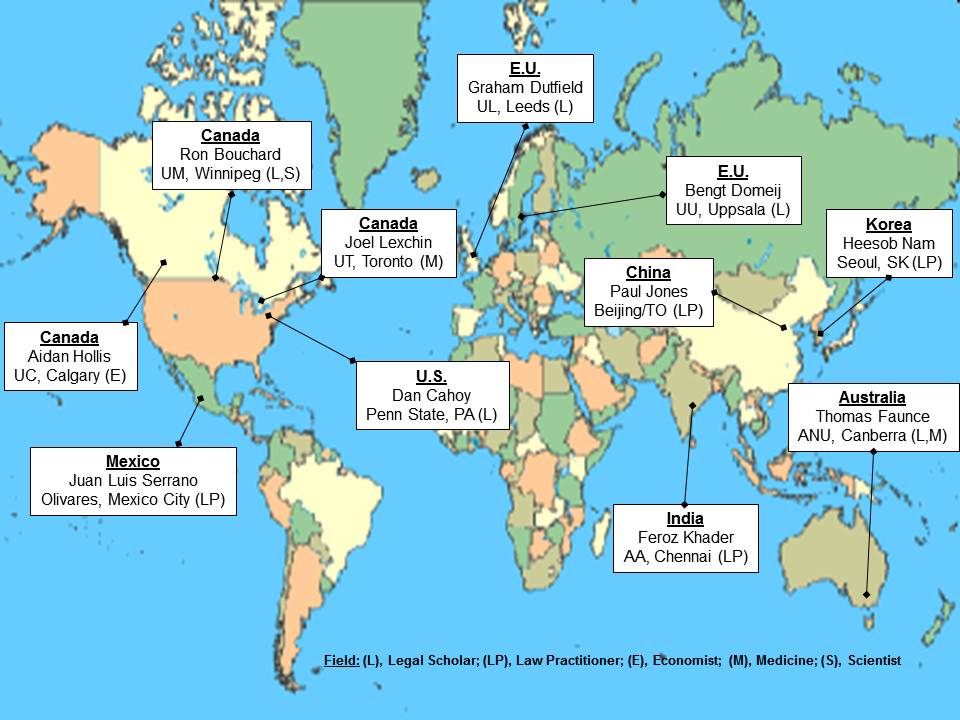
I am pleased to be part of a global consortium of eleven intellectual property law and health policy scholars, economists, and practicing lawyers in nine counties. The group is called the Consortium Study of Global Pharmaceutical Linkage.
The Consortium is spread across nations with established, maturing, and nascent linkage regulations as follows:
- Canada: Ron Bouchard (Manitoba)
- Canada: Aidan Hollis (Calgary)
- Canada: Joel Lexchin (Toronto)
- U.S.: Dan Cahoy (Penn State)
- EU: Bengt Domeij (Uppsala, Sweden)
- EU: Graham Dutfield (Leeds, U.K.)
- Mexico: Juan Louis Serrano (Mexico City)
- Australia: Tom Faunce (ANU)
- China: Paul Jones (Toronto)
- South Korea: Heesob Nam (Seoul)
- India: Feroz Ali Khader (Currently at Duke)
The Consortium includes individuals with litigation experience with pharmaceutical linkage regulations on both sides of the brand-generic divide, scholars in Faculties of Law, Medicine, Health, and Economics, and practicing lawyers working in law firms and Non-Governmental Organizations.
The Consortium is supported in its work by a Key Decision Maker Advisory Board composed of senior members of the judiciary and government in federal and provincial health, industry and intellectual property portfolios.
The goal of our work is to produce and use empirical knowledge relating to the structure and function of different global linkage regimes to assist key decision-makers in global governments and legal systems working with linkage regimes and related intellectual property law and policy.
UPDATE: The first paper from the Consortium has been accepted for publication
with
the
Minnesota Journal of Law, Science & Technology.
The full cite is:
Bouchard, R.A. Cahoy, D., Domeij, B., Dutfield, G., Faunce, T., Hollis, A., Jones, P., Ali Khader, F., Lexchin, J., Nam, H., & Serrano, J.L. "Structure-Function Analysis of Global Pharmaceutical Linkage Regulations."
Minnesota Journal of Law, Science & Technology. 12(2): 391-457. 2011.
The article is available on the journal website.